Life Sciences Survey Highlights Worries About Data Sprawl, Security
Organizations in the life sciences industry need to maintain regulated data in compliance with a number of global data privacy laws. Ideally, compliance is automatically ensured, and data is easily categorized. But we all know that this is not always the case in a decentralized, dynamic environment. So, how are the leading biotechs efficiently and securely managing collaboration and data?
Egnyte commissioned GatePoint Research to query 152 executives in Regulatory Affairs, R&D, Clinical and Quality about the challenges facing data-driven, mission-critical companies as they try to maintain GxP compliance, specifically around data integrity and data privacy and security.
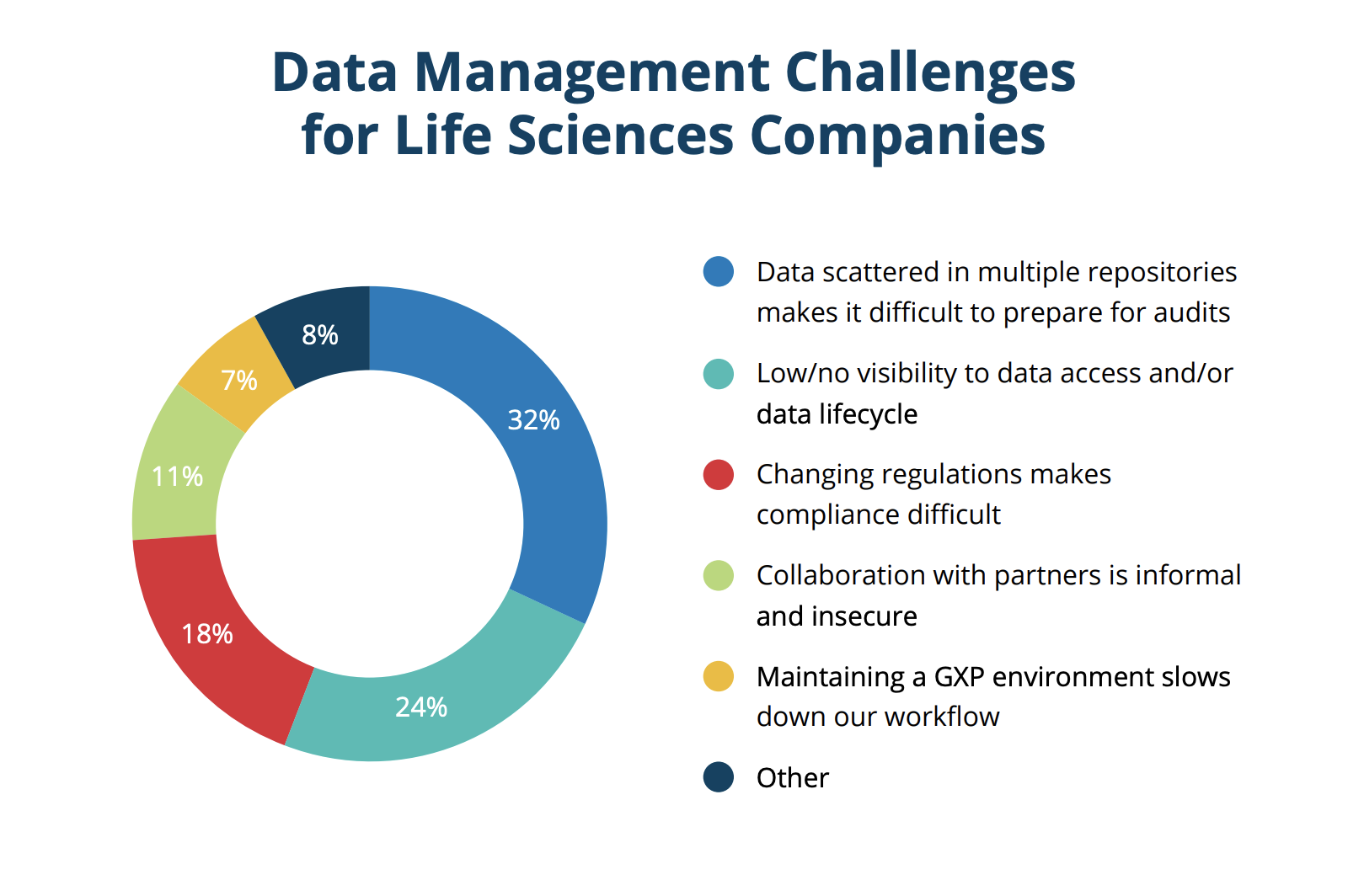
When considering data integrity, one of the most interesting things the study found is that the most popular applications used to manage regulated data are not designed for life sciences specifically. The most common applications to host regulated data, with 70% of organizations reporting they use them, were Microsoft platforms (365, Sharepoint, or Azure); although they're not designed for GxP guidelines, meaning they require strict manual procedures to satisfy compliance. Next was email at 46%, a technology that is neither secure nor auditable to FDA standards. In fact, the vast majority of technologies used for managing regulated data are not validated for managing life sciences data, which increases the risk of noncompliance and fines.
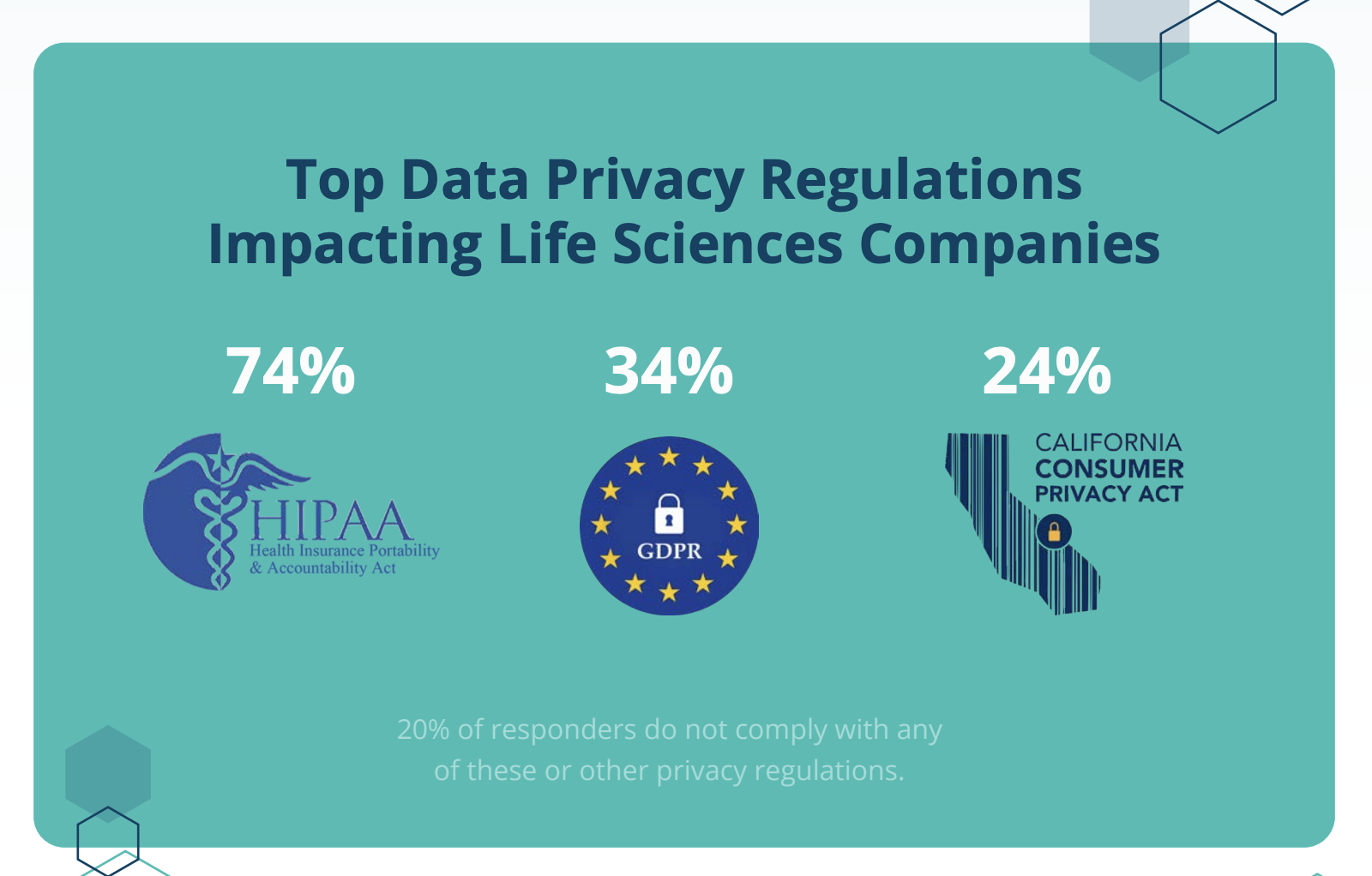
Protecting data integrity is just one part of GxP-compliant data management. Data privacy and security is a big concern as well. With 78% of organizations employing access controls and 63% encrypting data, privacy and security is a high-priority for life science organizations. However, fewer than half (45%) have active ransomware protections in place to prevent breaches and data loss. Ransomware has been an increasing risk in the last two years, with the FBI repeatedly releasing warnings that healthcare and biotech companies are targets.
The results of the study confirm a stark divide is developing amongst life science organizations, in effect creating two “classes.” The first class is made up of technology adopters leveraging software to automate tedious tasks like creating audit trails, monitoring for PII, and classifying and organizing documents and data, which allows them to keep pace with the internal speed of discovery. The second group consists of organizations that still operate from manual processes, leading to friction in collaborating with partners and difficulty maintaining compliance with regulations. The pandemic has accelerated experimentation with digital technologies, and we predict that organizations embracing digital transformation will see over-indexed growth in the coming years.
Check out the infographic to see the key results
Egnyte for Life Sciences is a unified platform for life sciences documents and data. The advanced platform supports features designed to maintain GxP compliance, securely share documents, and meet data privacy regulations. Customers benefit from managing regulated data, secure collaboration, and automated compliance with global data privacy laws. More than 16,000 businesses, and more than 600 life science organizations, trust Egnyte to provide visibility and control into their most valuable asset, their data. For more information, visit www.egnyte.com/lifesciences/